ตำรายาของประเทศไทย
Thai Pharmacopoeia
สำนักยาและวัตถุเสพติด กรมวิทยาศาสตร์การแพทย์ กระทรวงสาธารณสุข
Bureau of Drug and Narcotic, Department of Medical Sciences, Ministry of Public HealthCamomile Flowers; Chamomile; German Chamomile Flowers
Thai name ดอกหญ้าหอม (DOK YAA HOM)
Category Anti-inflammatory.
Chamomile Flowers consist of the dried flower heads of Matricaria chamomilla L. (Matricaria recutita L., Chamomilla recutita (L.) Rauschert) (Family Asteraceae alt. Compositae). They contain not less than 0.4 per cent v/w of blue volatile oil.
Origin of plant The plant of chamomile flowers is native to northern Europe and grows wildly in central European countries, especially abundant in eastern Europe. It is also found in western Asia, the Mediterranean region of northern Africa, and the United States of America.
Constitutents Chamomile flowers contain the blue volatile oils which yield terpenoids, a-bisabolol (levomenol) and its oxides, and azulenes including chamazulene and guaiazulene. They also contain matricin, en-yne-dicycloether, coumarins, and flavonoid glucosides such as apigenin-7-glucoside.
Description Odour, strong, pleasant, characteristic; taste, bitter and aromatic.
Macroscopical Flower head hemispherical, about 6 mm in diameter, consisting of a few ray florets and numerous disk florets on a receptacle with surrounding involucre. Involucre 2 to 3 rows of imbricated bracts; bract lanceolate, with blunt apex and scarious whitish edges, green, glabrous. Ray floret comprising 10 to 20 pistils, caducous; corolla about 6 mm long, about 2 mm wide, 3-toothed, traversed by 4 major veins, white becoming darken, ligulate. Disk floret perfect, about 2 mm long, yellow; corolla tubular, 5-toothed; stamens 5, epipetalous, syngenesious. Receptacle hemispherical when young, becoming conical in old flower head, hollowed, 3 to 10 mm in diameter; paleae absent. Fruit, if found, achene, ovoid, 3- to 5-longitudinally ribbed.
Microscopical
Involucral bract Outer, abaxial epidermis, a single layer of radially elongated cells; central part composed of chlorophyll tissue covered by elongated epidermal cells with sinuous lateral walls, stomata and secretory trichomes. Vascular bundle surrounded by numerous elongated, pitted sclereids with fairly large lumens.
Ligulate and tubular corollas Surface view shows isodiametric or elongated cells with wavy walls and a few glandular trichomes.
Ligulate florets Outer epidermis comprising papillary cells with striations radiating from the apex. Mesophyll containing very small clusters of calcium oxalate crystals; four major veins longitudinally running through the entire mesophyll, occasionally accompanied by 1 or 2 other shorter veins running in parallel to the major veins; each of the two median veins splitting into two near the apex and, with the lateral veins, anastomosing two by two to form 3 arcs at the three major terminal teeth of the ligule. Ovary oval to globose, bearing a sclerous ring consisting of a layer of cells at the base; epidermis of the ovary composed of elongated cells with sinuous walls bearing secretory trichomes between the walls; numerous, very small clusters of calcium oxalate are found. Stigmata bearing papillose epidermal cells at the ends. Pollen grains rounded and triangular, about 30 µm in diameter, with 3 germinal pores and spiny exile.
Packaging and storage Chamomile Flowers shall be kept in well-closed containers, protected from light and stored at a temperature not exceeding 25°.
Identification
A. Carry out the test as described in the “Thin-Layer Chromatography” (Appendix 3.1).
Standard solution A solution containing 1.0 mg per mL of borneol, 2.0 mg per mL of bornyl acetate, and 400 µg per mL of guaiazulene in toluene.
Test solution Reduce 1.0 g of the sample to a coarse powder, using a porcelain pestle and mortar. Transfer to a 1.5-cm × 15-cm chromatographic column, and tamp lightly with a short length of rubber hose. Rinse the pestle and mortar twice, each time with 10 mL of dichloromethane. Pour the rinsings into the column. Collect the percolate in a flask with a long, narrow neck, and remove the solvent by evaporation on a water-bath. Dissolve the residue in 0.5 mL of toluene.
Adsorbent Silica gel GF254
Mobile phase Chloroform
Application Apply 10 µL of each solution, as
20-mm bands.
Development and drying Develop the plate and dry in air.
Detection Examine the plate under ultraviolet light (254 nm). Spray the plate with anisaldehyde TS, heat at 105° for 5 to 10 minutes and examine under visible light. Observe the result.
Results When examined under ultraviolet light (254 nm), the test solution shows a largest quenching band, due to en-yne-dicycloether, corresponding in Rf to the band due to bornyl acetate in the standard solution; a quenching band due to matricin near the line of application. Several quenching bands are also observed.
When examined under visible light, the standard solution shows a brownish yellow band, that becomes violet-grey after a few hours, due to borneol in the lower third; a yellowish brown to grey band due to bornyl acetate in the middle; and a deep red band with a blue edge due to guaiazulene in the upper third of the chromatogram. The test solution shows a blue band due to matricin near the line of application; one violet-red band due to bisabolol, at Rf value between those of borneol and bornyl acetate bands; red bands of terpenes at Rf values similar to that of the guaizulene band obtained from the standard solution. Other violet-red bands are also present.
B. Dissolve 250 mg of 4-dimethylaminobenzal-dehyde in a mixture of 5 mL of phosphoric acid, 45 mL of acetic acid, and 45 mL of water. Transfer 2.5 mL of this solution and 0.1 mL of Test solution, to a test tube. Heat on a water-bath for 2 minutes, and allow to cool. Add 5 mL of solvent hexane, and shake: the aqueous layer has a distinct greenish blue or blue colour.
Foreign matter Not more than 2.0 per cent w/w (Appendix 7.2).
Acid-insoluble ash Not more than 1.5 per cent w/w (Appendix 7.6).
Total ash Not more than 13.0 per cent w/w (Appendix 7.7).
Apigenin-7-glucoside content Not less than 0.3 per cent w/w. Carry out the test as described in the “Liquid Chromatography” (Appendix 3.5).
Mobile phase:
Dilute phosphoric acid Mix 5.0 mL of phosphoric acid in 50 mL of water. Dilute with water to 100 mL.
Mobile phase A Prepare 0.005 M potassium dihydrogenphosphate. Adjust with Dilute phosphoric acid to a pH of 2.55±0.05.
Mobile phase B Acetonitrile and methanol (13:7)
The step gradient of mobile phases is as follows:
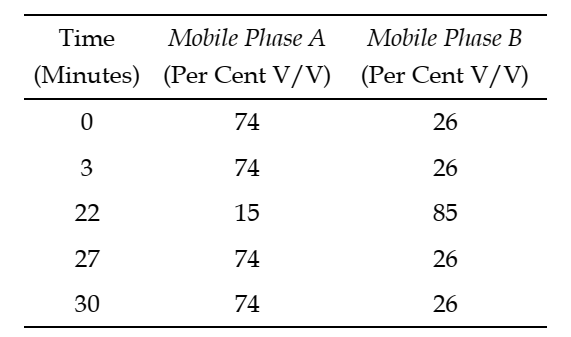
Standard solution A solution of 25.0 µg per mL of Apigenin-7-glucoside RS and 10.0 µg per mL of 7-methoxycoumarin in methanol and water (1:1).
Test solution Transfer about 1 g, accurately weighed, to a suitable flask fitted with a reflux condenser and a stirrer. Add 80.0 mL of methanol, and reflux the mixture with stirring for 1 hour. Cool the flask to room temperature, pass the extract through a folded filter paper, and collect the filtrate in a 100-mL volumetric flask. Rinse the flask with 3 mL of methanol, pour the methanolic rinsings through the filter paper, and add the filtrate to the volumetric flask. Dilute with methanol to volume, and mix. Transfer 25.0 mL of the filtered solution to a round-bottomed flask fitted with a reflux condenser and a stirrer. Add 5.0 mL of sodium hydroxide TS and reflux the mixture for 25 minutes. Cool the flask and adjust the solution with hydrochloric acid to a pH of 5.0 to 6.2. Quantitatively transfer the solution to a 50-mL volumetric flask, dilute with methanol to volume and filter, discarding the first 10 mL of the filtrate.
Chromatographic system
DETECTOR Ultraviolet light (335 nm)
COLUME A stainless steel column (12.5 cm × 4.6 mm), packed with octadecylsilane chemically bonded to porous silica or ceramic microparticles (5 µm).
Column temperature 43°±1°
Flow rate 1.0 mL per minute. (Note Make adjustments, if necessary, to obtain relative retention times of 0.63 for apigenin-7-glucoside and 1.0 for 7-methoxycoumarin.)
System suitability
Sample Standard solution (Note The relative retention times for apigenin-7-glucoside, 7-methoxycoumarin, apigenin, trans-spiroether, and cis-spiroether are about 0.63, 1.0, 1.2, 1.6, and 1.8, respectively.)
Suitability requirements
Resolution Not less than 3.5 between apigenin-7-glucoside and 7-methoxycoumarin.
Relative standard deviation Not more than 2.0 per cent for apigenin-7-glucoside.
Procedure Separately inject equal volumes (about 15 µL) of Standard solution and Test solution into the chromatograph and measure the responses for the major peaks. (Note Allow the test solution to elute for not less than 6 times the retention time of apigenin-7-glucoside.
Calculation Calculate the content of C21H20O10 in the portion of the Chamomile Flowers taken, using the declared content of C21H20O10 in Apigenin-7-glucoside RS.
Bisabolane derivatives content Not less than 0.15 per cent w/w. Carry out the test as described in the “Gas Chromatography” (Appendix 3.4).
Standard solution A solution containing 1 mg per mL of Levomenol RS in cyclohexane.
Test solution Transfer the volatile oils obtained in the Assay to a 25-mL volumetric flask, rinse the graduated tube of the apparatus with a small portion of cyclohexane, transfer the rinsing to the 25-mL volumetric flask, add cyclohexane to volume, and mix.
Chromatographic system
DETECTOR Flame ionization
COLUMN A fused-silica capillary column (30 m × 0.32 mm), coated with a 0.25-µm film of polyethylene glycol compound (average molecular weight about 15,000)
TEMPERATURE
Column Initial Temperature 70°
Temperature Ramp 4° per minute
Final Temperature 230°
Hold Time at Final Temperature 10 minutes
Detector 250°
Injection port 220°
CARRIER GAS Helium
FLOW RATE 1.0 mL per minute
System suitability
Sample Standard solution
Suitability requirements
Symmetry factor Not more than 1.8 for levomenol
Relative standard deviation Not more than 2.0 per cent determined from the chamomile peak
Procedure Separately inject equal volumes (about 1 µL) of Standard solution and Test solution into the chromatograph and measure the responses for the major peaks. Identify the peaks due to levomenol, bisabolol oxide B, bisabolol oxide, and bisabolol oxide A in the test solution, using the retention time of levomenol in the standard solution and the approximate relative retention times of 0.89, 0.97, and 1.1 for bisabolol oxide B, bisabolol oxide, and bisabolol oxide A, respectively, with reference to the levomenol (bisabolol) peak.
Calculation Calculate the content of bisabolane derivatives (bisabolol oxide B, bisabolol oxide, and bisabolol oxide A) in the portion of the Chamomile Flowers taken, calculated as levomenol.
Assay Carry out the “Determination of Volatile Oil” (Appendix 7.3). Use 60 g, in coarse powder, freshly prepared and accurately weighed. Use 300 mL of water as the distillation liquid and a 2-L round-bottomed flask. Distil at a rate of 3 to 4 mL per minute for 5 hours. Use 0.5 mL of xylene in the graduated tube. Calculate the content of volatile oil, in mL, in the portion of the Chamomile Flowers taken. (Note Retain the volatile oils for use in the test for Bisabolane derivatives content.)