ตำรายาของประเทศไทย
Thai Pharmacopoeia
สำนักยาและวัตถุเสพติด กรมวิทยาศาสตร์การแพทย์ กระทรวงสาธารณสุข
Bureau of Drug and Narcotic, Department of Medical Sciences, Ministry of Public Health
C19H16Cl2N3O5S·Na·H2O 510.32 13412-64-1
4-Thia-1-azabicyclo[3.2.0]heptane-2-carboxylic acid, 6-[[[3-(2,6-dichlorophenyl)-5-methyl-4-isoxazolyl] carbonyl]amino]-3,3-dimethyl-7-oxo-, [2S-(2,5,6β)]-, monosodium salt, monohydrate.
Anhydrous 492.30 343-55-5
Category Antibacterial (of the penicillin class).
Dicloxacillin Sodium contains not less than 850 µg of dicloxacillin, C19H17Cl2N3O5S, per mg.
Description White to off-white, crystalline powder.
Solubility Freely soluble in water.
Contra-indication It is contra-indicated in patients who have shown hypersensitivity to any member of the penicillins or of the cephalosporins or other related allergens.
Warning
1. Hypersensitivity reactions are the most common adverse effects noted with the penicillins. Serious and occasionally fatal hypersensitivity (anaphylactoid) reactions may occur, regardless of the route of administration or the dose.
2. Serious superinfections with resistant organisms, especially gram-negative bacteria (e.g., Pseudomonas and Proteus) and Candida, may occur following long-term therapy with penicillins.
3. Massive doses of penicillins may cause hematologic abnormalities (including anemia, thrombocytopenia, leukopenia, neutropenia, and eosinophilia).
4. Clostridium difficile colitis may develop in some patients.
5. Concurrent use with any bacteriostatic drug (e.g., tetracyclines, chloramphenicol, etc.) is not recommended for the treatment of meningitis or other situations where a rapid bactericidal effect is necessary.
Precaution Periodic assessment of renal, hepatic, and hematopoietic systems is recommended during therapy.
Additional information
1. Dicloxacillin Sodium should not be used in the treatment of meningitis since it penetrates poorly into cerebrospinal fluid.
2. Group A beta-hemolytic streptococcal infections should be treated for at least 10 days to help prevent the development of acute rheumatic fever or acute glomerulonephritis.
Packaging and storage Dicloxacillin Sodium shall be kept in tightly closed containers and stored at a temperature not exceeding 25º.
Labelling The label on the container states (1) the expiration date; (2) the storage condition.
Identification
The infrared absorption spectrum is concordant with the spectrum obtained from Dicloxacillin Sodium RS (Appendix 2.1) or with the reference spectrum of Dicloxacillin Sodium.
The retention time of the major peak of the Assay preparation corresponds to that of the Standard preparation, as obtained in the Assay.
It yields the reactions characteristic of sodium salts (Appendix 5.1).
pH 4.5 to 7.5, in a 1.0 per cent w/v solution (Appendix 4.11).
Water Not less than 3.0 per cent w/w and not more than 5.0 per cent w/w (Karl Fischer Method, Appendix 4.12).
N,N-Dimethylaniline Not more than 20 ppm. (Appendix 5.16)
Organic impurities Carry out the test as described in the “Liquid Chromatography” (Appendix 3.5). (Note Protect solutions containing dicloxacillin from light.
Diluent, System suitability stock solution, Mobile phase A, Mobile phase B, and Chromatographic System Proceed as directed in the Assay.
System suitability solution A solution containing
10 µg per mL of Dicloxacillin Related Compound D RS from System suitability stock solution and 1 mg per mL of Dicloxacillin Sodium RS in Diluent. Store this solution at 4°.
Standard solution A solution containing 10 µg per mL of Dicloxacillin Sodium RS in Diluent. Sonicate as needed to dissolve. Store this solution at 4°.
Test solution Weigh accurately a suitable quantity of Dicloxacillin Sodium, dissolve in and dilute with Diluent to obtain a solution containing 1 mg per mL of dicloxacillin sodium. Sonitcate as needed to dissolve. Store this solution at 4º.
Mobile phase
The step gradient of mobile phases is as follows:
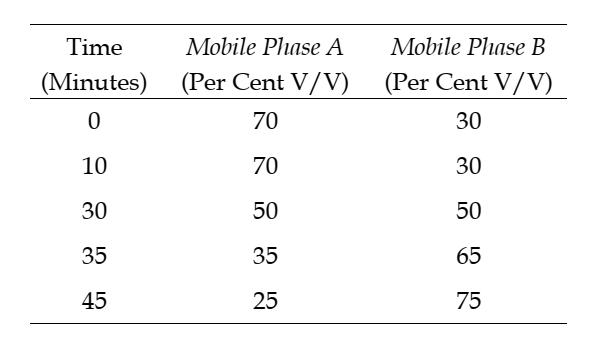
Return to the original conditions and re-equilibrate the system.
(Note The relative retention times for dicloxacillin and dicloxacillin related compound D are about 1.0 and 1.1, respectively.)
System Suitability
Sample System suitability solution and Standard solution.
Suitability requirements
Symmetry factor Between 0.8 and 1.5 for the dicloxacillin peak in the chromatogram of the standard solution.
Resolution Not less than 1.5 between dicloxacillin related compound D and dicloxacillin peaks in the chromatogram of the system suitability solution.
Relative standard deviation Not more than
2.5 per cent for the dicloxacillin peak in the chromatogram of the standard solution.
Procedure Separately inject equal volumes (about
10 mL) of Standard solution and Test solution into the chromatograph, record the chromatograms, and measure the responses for the major peaks.
Calculation Calculate the percentage of each impurity in the portion of the Dicloxacillin Sodium taken, with reference to the relative retention times and relative response factors as described in the following table using the expression:
(ru/rs) × (Cs/Cu) × P × (F1/F2) × 100
where ru = peak response of each impurity from the Test solution
rs = peak response from the Standard solution
Cs = concentration, in mg/mL, of Dicloxacillin Sodium RS in the Standard solution
Cu = concentration, in mg/mL, of Dicloxacillin Sodium in the Test solution
P = potency, in µg/mg, of dicloxacillin in Dicloxacillin Sodium RS
F1 = conversion factor, 0.001 mg/µg
F2 = relative response factor (see Table 1)
(Note The reporting threshold is 0.05 per cent).
Limits
— individual or any other impurities: not more than 1.0 per cent,
—total impurities: not more than 5.0 per cent.
Assay Carry out the determination as described in the “Liquid Chromatography” (Appendix 3.5). (Note Protect solutions containing dicloxacillin from light.
Diluent Acetonitrile and water (50:50).
Standard preparation A solution containing 100 µg per mL of Dicloxacillin Sodium RS in Diluent. Sonicate as needed to dissolve. Store this solution at 4°.
System suitability stock solution A solution containing 100 µg per mL of Dicloxacillin Related Compound D in Diluent. Sonicate as needed to dissolve.
System suitability solution A solution containing 1 µg per mL of Dicloxacillin Related Compound D RS from System suitability stock solution and 100 µg per mL of Dicloxacillin Sodium RS in Diluent. Store this solution at 4°.
Assay preparation Weigh accurately a suitable quantity of Dicloxacillin Sodium, dissolve in and dilute with Diluent to obtain a solution containing 100 µg per mL of dicloxacillin sodium. Sonicate as needed to dissolve. Store this solution at 4º.
Mobile phase
MOBILE PHASE A Prepare a solution containing 1.18 mg per mL of sodium 1-hexanesulfonate monohydrate and 0.8 µL per mL of ammonium hydroxide; adjusted with phosphoric acid to a pH of 2.9 to 3.1.
MOBILE PHASE B Acetonitrile.
The step gradient of mobile phases is as follows:

Return to the original conditions and re-equilibrate the system.
Chromatographic System
Detector Ultraviolet light (225 nm).
Column A stainless steel column (25 cm × 4.6 mm), packed with octadecylsilane chemically bonded to porous silica or ceramic microparticles (5 µm).
Temperatures
Autosampler 4º.
Column 40º.
Flow rate 1.5 mL per minute.
(Note The relative retention times for dicloxacillin and dicloxacillin related compound D are about 1.0 and 1.1, respectively.)
System Suitability
Sample System suitability solution and Standard preparation.
Suitability requirements
Symmetry factor Between 0.8 and 1.5 for the dicloxacillin peak in the chromatogram of the standard preparation.
Resolution Not less than 1.5 between dicloxacillin and dicloxacillin related compound D peaks in the chromatogram of the system suitability solution.
Relative standard deviation Not more than 0.73 per cent for the dicloxacillin peak in the chromatogram of the standard preparation.
Procedure Separately inject equal volumes (about 10 mL) of Standard preparation and Assay preparation into the chromatograph, record the chromatograms, and measure the responses for the major peaks.
Calculation Calculate the content of C19H17Cl2N3O5S in the portion of the Dicloxacillin Sodium taken, using the declare content of C19H17Cl2N3O5S in Dicloxacillin Sodium RS.
