ตำรายาของประเทศไทย
Thai Pharmacopoeia
สำนักยาและวัตถุเสพติด กรมวิทยาศาสตร์การแพทย์ กระทรวงสาธารณสุข
Bureau of Drug and Narcotic, Department of Medical Sciences, Ministry of Public Health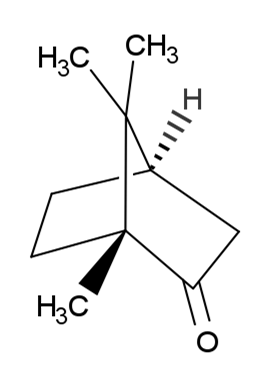
C10H16O 152.24 464-49-3
(1R,4R)-1,7,7-Trimethylbicyclo[2.2.1]heptan-2-one.
Category Counter-irritant; pharmaceutic aid; antipruritic.
Natural Camphor is a ketone obtained from Cinnamomum camphora (L.) J. Presl (Family Lauraceae) and purified by sublimation.
Description Colourless, transparent crystals, crystalline masses, blocks of tough consistence or crumbly masses known as flowers of camphor; odour, strong and characteristic.
Solubility Slightly soluble in water, very soluble in ethanol and light petroleum, freely soluble in fixed oils, very slightly soluble in glycerol.
Other relevant information
1. Local application to the nostrils of infants may cause immediate collapse.
2. Liniment containing camphor should not be applied to broken or inflamed skin, mucous membranes, or near the eyes. It readily volatilizes at ordinary temperature.
Packaging and storage Natural Camphor shall be kept in tightly closed containers, and stored at a temperature not exceeding 25°.
Labelling The label on the container indicates whether it is obtained from natural sources or is prepared synthetically.
Identification
A. The infrared absorption spectrum is concordant with the spectrum obtained from Racemic Camphor RS or with the reference spectrum of Racemic Camphor.
B. It complies with the test for Optical rotation.
C. Dissolve 1 g in 30 mL of methanol, add 1 g of hydroxylamine hydrochloride and 1 g of anhydrous sodium acetate, boil under a reflux condenser for 2 hours, and allow to cool. Add 100 mL of water, filter, wash the precipitate with 10 mL of water, and recrystallize from 10 mL of a mixture of 4 volumes of ethanol and 6 volumes of water: the crystals, after drying over phosphorus pentoxide desiccant at a pressure of 1.5 to 2.2 kPa (about 11 to 19 Torr), melt between 118° and 121° (Appendix 4.3).
Melting range 174° to 181° (Appendix 4.3), but using a capillary tube with an internal diameter of not greater than 2 mm.
Optical rotation +40° to +43° at 20°, determined in a 10.0 per cent w/v solution in ethanol (Appendix 4.8).
Water Dissolve 1 g in 10 mL of petroleum ether (boiling range, 40° to 60°): the solution is clear (Appendix 4.1).
Halogens Not more than 100 ppm. To 10.0 mL of a 10.0 per cent w/v solution in 2-propanol in a distillation flask, add 1.5 mL of 2 M sodium hydroxide and 50 mg of Raney nickel catalyst, heat on a water-bath until the solvent has evaporated and allow to cool. Add 5 mL of water, mix, filter through a wet filter paper previously washed with water until free from chloride, and dilute the filtrate to 10.0 mL with water. To 5.0 mL of this solution add nitric acid dropwise until the precipitate that is produced dissolves and add sufficient water to produce 20 mL. The resulting solution shows no more chloride than that corresponds to 0.07 mL of 0.020 M hydrochloric acid.
Related substances Carry out the test as described in the “Gas Chromatography” (Appendix 3.4).
Standard solution (a) Dilute 1.0 mL of Test solution to 100.0 mL with heptane.
Standard solution (b) Dilute 10.0 mL of Standard solution (a) to 20.0 mL with heptane.
Standard solution (c) Dissolve 500 mg of borneol in heptane and dilute to 25.0 mL with the same solvent. Dilute 5.0 mL of the solution to 50.0 mL with heptane.
Standard solution (d) Dissolve 50 mg of linalool and 50 mg of bornyl acetate in heptane and dilute to 100.0 mL with the same solvent.
Test solution Dissolve 2.5 g of the sample in heptane and dilute to 25.0 mL with the same solvent. Chromatographic system.
Detector Flame ionization
Column A glass column (30 m × 0.25 mm) packed with macrogol 20000 (0.25 µm).
Temperature
Column The temperature programme is as follows:
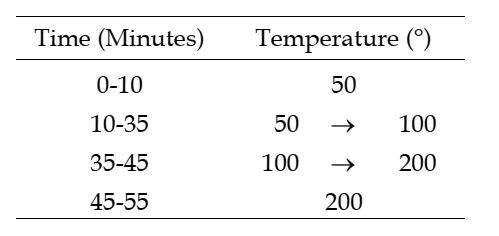
Injection port 220°
Detector 250°
Carrier gas Helium
Split ratio 1:70
Flow rate 45 cm per second (1.3 mL per minute)
System suitability
Sample Standard solution (d)
Suitability requirements
Resolution Not less than 3.0 between bornyl acetate and linalool peaks.
Procedure Inject a portion (about 1 mL) of Standard solution (a), Standard solution (b), Standard solution (c), Standard solution (d), and Test solution into the chromatograph. Measure the area of the principal peak in each chromatogram.
Limits
— borneol: not more than the area of the principal peak in the chromatogram obtained from standard solution (c) (2.0 per cent).
— any impurity: not more than half of the area of the principal peak in the chromatogram obtained from standard solution (a) (0.5 per cent).
— total of other impurities: not more than 4 times the area of the principal peak in the chromatogram obtained from standard solution (a) (4.0 per cent).
— disregard limit: 0.1 times the area of the principal peak in the chromatogram obtained from standard solution (b) (0.05 per cent).
Non-volatile matter Not more than 0.1 per cent w/w when volatilized at 105° to constant weight; use about
5 g, accurately weighed.
Assay Carry out the determination as described in the “Gas Chromatography” (Appendix 3.4).
Internal standard preparation Dissolve about 2 g of methyl salicylate, accurately weighed, in 50.0 mL of absolute ethanol.
Standard preparation Weigh accurately about 100 mg of Natural Camphor RS, add 5.0 mL of Internal standard preparation, dissolve in absolute ethanol and dilute to 100.0 mL with the same solvent.
Assay preparation Weigh accurately about 100 mg of Natural Camphor, add 5.0 mL of Internal standard solution, dissolve in absolute ethanol and dilute to
100.0 mL with the same solvent.
Chromatographic system
Detector Flame ionization
Column A glass column (3 m × 3 mm) packed with 10 per cent of polyethylene glycol 20 M on silanized diatomaceous support 180- to 250-mesh.
Temperature
Column 160°
Carrier gas Nitrogen
Flow rate Adjust so that the retention time of natural camphor is about 6 minutes.
System suitability
Sample Standard preparation
Suitability requirements
Resolution Not less than 7 between natural camphor and methyl salicylate peaks.
Relative standard deviations Chromatog-raph six injections of Standard preparation, and record peak areas. The analytical system is suitable for conducting this Assay if the relative standard deviation for the area ratios of natural camphor to methyl salicylate is not more than 1.0 per cent.
Procedure Separately inject equal volumes (about 2 µL) of Standard preparation and Assay preparation into the chromatograph, record the chromatograms and measure the areas of natural camphor and methyl salicylate peaks. Calculate the area ratios of natural camphor to methyl salicylate peaks.
Calculation Calculate the content of C10H16O in the portion of Natural Camphor taken, using the declared content of C10H16O in Natural Camphor RS.