ตำรายาของประเทศไทย
Thai Pharmacopoeia
สำนักยาและวัตถุเสพติด กรมวิทยาศาสตร์การแพทย์ กระทรวงสาธารณสุข
Bureau of Drug and Narcotic, Department of Medical Sciences, Ministry of Public Health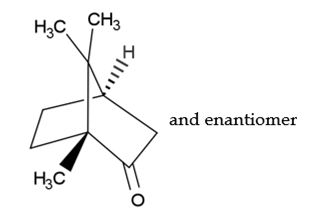
C10H16O 152.24 76-22-2
(1RS,4RS)-1,7,7-Trimethylbicyclo[2.2.1]heptan-2-one.
Category Counter-irritant; pharmaceutic aid; antipruritic.
Description White, crystalline powder or friable, crystalline masses; odour, strong and characteristic.
Solubility Slightly soluble in water, very soluble in ether and in petroleum ether; freely soluble in fixed oils; soluble in 1 part of ethanol; very slightly soluble in glycerol.
Other relevant information; Packaging and storage; Labelling See under Camphor, Natural.
Identification; Halogens Comply with the tests as described under Camphor, Natural.
Clarity and colour of solution A 10.0 per cent w/v solution in ethanol is clear (Appendix 4.1) and colourless.
Acidity or alkalinity To a 10.0 per cent w/v solution in ethanol add 0.1 mL of phenolphthalein TS. The solution is colourless and not more than 0.20 mL of 0.10 M sodium hydroxide is required to change the colour of the solution.
Water Dissolve 1 g in 10 mL of petroleum ether (boiling range, 50° to 70°): the solution is clear (Appendix 4.1).
Optical rotation -0.15° to +0.15°, determined in a 10.0 per cent w/v solution in ethanol (Appendix 4.8).
Related substances Carry out the test as described in the “Gas Chromatography” (Appendix 3.4).
Standard solution (a) Dissolve 50 mg of the sample and 50 mg of bornyl acetate in hexane and dilute to 50.0 mL with the same solvent.
Standard solution (b) Dilute 1.0 mL of the test solution to 200.0 mL with hexane.
Test solution Dissolve 50 mg of the sample in hexane and dilute to 50.0 mL with the same solvent.
Chromatographic system
Detector Flame ionization
Column A glass column (2 m × 2 mm) packed with macrogol 20000 (0.25 µm)
Temperature
Column 130°
Injection port 200°
Detection 200°
Carrier gas Nitrogen
Split ratio 1:70
Flow rate 30 mL per minute
System suitability
Sample Standard solution (a)
Suitability requirements
Resolution Not less than 1.5 between the peaks due to camphor and bornyl acetate in the chromatogram obtained with Standard solution (a).
Signal-to-noise ratio Not less than 5 for the principal peak in the chromatogram obtained with Standard solution (b).
Procedure Inject a portion (about 1 mL) of Standard solution (a), Standard solution (b) and Test solution into the chromatograph. Measure the area of the principal peak in each chromatogram.
Limits
— any impurity: for each impurity, not more than 2 per cent of the area of the principal peak,
— total of other impurities: not more than 4 per cent of the area of the principal peak,
— disregard limit: any peak areas less than that of the principal peak in the chromatogram obtained from standard solution (b).
Non-volatile matter Not more than 0.05 per cent w/w. Evaporate about 2 g, accurately weighed, to dryness on a water-bath and heat at 100° to 105° for 1 hour.
Assay Carry out the determination as described in the “Gas Chromatography” (Appendix 3.4).
Internal standard preparation and Chromatographic system Proceed as directed in the Assay under Natural Camphor.
Standard preparation Weigh accurately about
100 mg of Racemic Camphor RS, add 5.0 mL of Internal standard preparation, dissolve in absolute ethanol and dilute to 100.0 mL with the same solvent.
Assay preparation Weigh accurately about 100 mg of Racemic Camphor, add 5.0 mL of Internal standard solution, dissolve in absolute ethanol and dilute to
100.0 mL with the same solvent.
Suitability requirements
Resolution Not less than 7 between racemic camphor and methyl salicylate peaks.
Relative standard deviations Chromatograph six injections of Standard preparation, and record peak areas. The analytical system is suitable for conducting this Assay if the relative standard deviation for the area ratios of racemic camphor to methyl salicylate is not more than 1.0 per cent.
Procedure Separately inject equal volumes (about
2 µL) of Standard preparation and Assay preparation into the chromatograph, record the chromatograms and measure the areas of racemic camphor and methyl salicylate peaks. Calculate the area ratios of racemic camphor to methyl salicylate peaks.
Calculation Calculate the content of C10H16O in the portion of Racemic Camphor taken, using the declared content of C10H16O in Racemic Camphor RS.